Abstract
INTRODUCTION: Nodal marginal zone lymphoma (nMZL) is a rare lymphoma accounting for < 2% of all non-Hodgkin lymphomas. As 1 of 3 recognized MZL subtypes, nMZL has only been formally recognized by the World Health Organization Classification in the last 2 decades. Consequently, characterization is limited. Therefore, we reviewed a large cohort with the goal of defining characteristics, survival, and time-to-event descriptions.
METHODS: Using a search of the Memorial Sloan Kettering (MSK) Lymphoma Outcomes Database, we identified patients with nMZL between 1/1/2001-6/1/2021. Only patients with a histological diagnosis based on pathology review at MSK were included. Patients with splenic involvement were included if presentation was predominantly nodal on radiographic review. Patients with nodal and extranodal involvement that was indistinguishable between nMZL and non-nodal MZL were excluded. Data were collected by chart review. Baseline Follicular Lymphoma International Prognostic Index (FLIPI) scores were calculated to evaluate impact on prognosis. Management strategies were classified into 1 of 5 categories (observation, radiation, rituximab/anti-CD20 monotherapy, chemoimmunotherapy, or other). Active treatment was considered any non-observation treatment. The primary objective was to determine the progression-free (PFS) and overall survival (OS) of the entire cohort, as estimated by the Kaplan-Meier method and compared for various features using the log-rank test. Time-to-1st and 2nd active treatment, as well as time-to-transformation, was evaluated by cumulative incidence function with death as a competing risk; 3 patients with transformed disease at initial presentation were excluded from time-to-transformation analyses.
RESULTS: We identified a total of 187 patients with nMZL. The median age at diagnosis was 62 (interquartile range: 50-69). Among those evaluable for baseline FLIPI (n=139), 61 (44%), 35 (25%), and 43 (31%) had scores of 0-1, 2, and ≥ 3. Initial management strategies varied; the most common approaches were observation (n=89, 48%) and chemoimmunotherapy (n=44, 24%), followed by rituximab/anti-CD20 monotherapy (n=26, 14%), radiation (n=18, 9.6%), and other (n=14, 5.3%), which predominantly consisted of chemotherapy prior to widespread use of anti-CD20 therapy. Forty-eight patients (28%) never required therapy. Patients who received observation as first management were more likely to have stage I/II (p<0.001) and FLIPI 0-1 (p=0.008) in comparison to others.
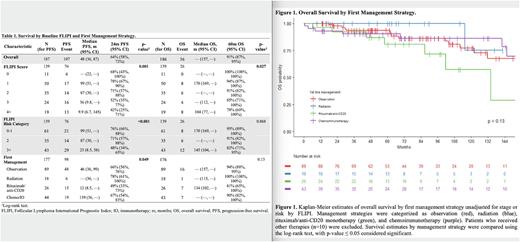
Among survivors, the median follow-up time was 70 months (m) (range 7-252); for the entire cohort, median PFS was 48 m (95% CI 36-87) and median OS was not reached (157 m-not reached). Overall 5-year PFS and OS was 47% (40%-56%) and 91% (87%-95%), and overall 10-year PFS and OS was 33% (25%-43%) and 76% (68%-85%). PFS and OS varied significantly by baseline FLIPI (Table 1), with 5-year PFS for FLIPI 0-1, 2, and ≥ 3 of 55%, 56%, and 30% (p<0.001), and 5-year OS for FLIPI 0-1, 2, and ≥ 3 of 46%, 40%, and 16% (p<0.001). By first management strategy, non-risk adjusted median 5-year PFS for observation, radiation, rituximab/anti-CD20 monotherapy, and chemoimmunotherapy was 45%, 65%, 34%, and 58% (p=0.049), and 5-year OS was 94%, 100%, 81%, and 90% (p=0.13) (Figure 1).
Among patients who ever received treatment (n=139), median time to 1st active treatment was 4.3 m (3.1-11). For those observed initially, the median time to active treatment was 72 m (49-not reached). For those who received at least 2 active treatments (n=60), the cumulative incidence of 2nd active treatment (in the presence of death as a competing risk) at 24 and 60 m was 36% and 46%, respectively. For those initially treated with radiation, rituximab/anti-CD20, or chemoimmunotherapy, the cumulative incidence of 2nd active therapy at 24 m was 6%, 44%, or 34%, respectively (p=0.02). Overall, 26 patients (14%) experienced transformation. For those who transformed after original presentation, cumulative incidence at 24, 60, and 120 m was 4%, 11%, and 14%.
CONCLUSIONS: To our knowledge, this is the largest cohort of nMZL with survival and time-to-event analyses. Initial management strategies varied, though observation was chosen in nearly half (48%) of patients, reflecting a commonly indolent presentation. Transformation was uncommon (14%). nMZL frequently has an indolent course and initial expectant observation is often a management option.
Disclosures
Batlevi:Bristol-Myers Squibb: Other: Ownership / Equity Interests; Provision of Services; ADC Therapeutics: Other: Provision of Services; Dava Oncology: Other: Provision of Services; Autolus: Research Funding; Bayer: Research Funding; Epizyme: Research Funding; Janssen: Research Funding; Novartis: Research Funding; Roche/Genentech: Research Funding; Xynomic: Research Funding; GLG Pharma: Consultancy; Juno/Celgene: Consultancy; Kite Pharma: Consultancy; Life Sciences: Consultancy; Seattle Genetics: Consultancy. Falchi:Roche: Consultancy, Research Funding; Genetech: Consultancy, Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding; Genmab: Consultancy, Research Funding. Horwitz:Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees; Affimed: Research Funding; C4: Research Funding; Verastem/SecuraBio: Research Funding; ONO Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Millennium /Takeda: Research Funding; Seattle Genetics,: Research Funding; Kyowa Hakko Kirin: Consultancy; Takeda: Consultancy; SecuraBio: Honoraria; Crispr Therapeutics: Research Funding; Shoreline Biosciences, Inc.: Membership on an entity's Board of Directors or advisory committees; Yingli Pharma Limited and Tubulis: Honoraria; Affimed,: Consultancy; ADC Therapeutics: Research Funding; Cimieo Therapeutics: Honoraria; Kyowa Hakko Kirin: Research Funding; Daiichi Sankyo: Research Funding. Lahoud:MorphoSys, Inc: Membership on an entity's Board of Directors or advisory committees. Lue:TG Therapeutics: Consultancy; Epizyme: Consultancy. Matasar:Pharmacyclics: Honoraria, Research Funding; ADC Therapeutics: Consultancy, Honoraria; Janssen: Honoraria, Research Funding; ImmunoVaccine Technologies: Honoraria, Research Funding; GlaxoSmithKline: Honoraria, Research Funding; Teva: Consultancy; Takeda: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria, Research Funding; Rocket Medical: Consultancy, Research Funding; Merck: Consultancy, Current equity holder in private company; Juno Therapeutics: Consultancy; Genentech, Inc.: Consultancy, Honoraria, Research Funding; F. Hoffmann-La Roche Ltd.: Consultancy, Honoraria, Research Funding; IMV Therapeutics: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; AstraZeneca: Consultancy; IGM Biosciences: Research Funding; Karyopharm: Consultancy; Bayer: Consultancy, Honoraria, Research Funding; TG Therapeutics: Consultancy; Daiichi Sankyo: Consultancy. Moskowitz:ADC Therapeutics: Research Funding; Biegene: Research Funding; Miragen: Research Funding; Seattle Genetics: Research Funding; Merck: Research Funding; Bristol-Myers Squibb: Research Funding; Incyte: Research Funding; SecuraBio: Research Funding; Affimed: Honoraria; Imbrium Therapeutics L.P./Purdue: Honoraria; Janpix Ltd: Honoraria; Merck: Honoraria; Seattle Genetics: Honoraria; Takeda: Honoraria. Noy:Janssen: Research Funding. Palomba:Ceramedix: Consultancy; BeiGene: Consultancy. Vardhana:Immunai: Membership on an entity's Board of Directors or advisory committees; Koch Disruptive Technologies: Consultancy. Salles:Roche/Genentech, Gilead Sciences, Janssen, Celgene, Novartis, MorphoSys AG, Epizyme, Alimera Sciences, Genmab, Debiopharm Group, Velosbio, Bristol-Myers Squibb, BeiGene, Incyte, Miltenyi Biotec, Ipsen, Kite, a Gilead Company, Loxo, Rapt: Consultancy; Roche/Genentech, Janssen, Celgene, Gilead Sciences, Novartis, AbbVie, MorphoSys AG, Amgen, Bayer, Epizyme, Regeneron, Kite, a Gilead Company: Honoraria; AbbVie, BeiGene, Bristol Myers Squibb, Celgene, Debiopharm, Epizyme, Genentech/Roche, Genmab, Incyte, Kite, a Gilead Company, Miltenyi, MorphoSys, Takeda, and VelosBio: Membership on an entity's Board of Directors or advisory committees. Straus:Takeda Pharmaceuticals: Consultancy; Seagen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal